Engineerblogger
Feb 22, 2012
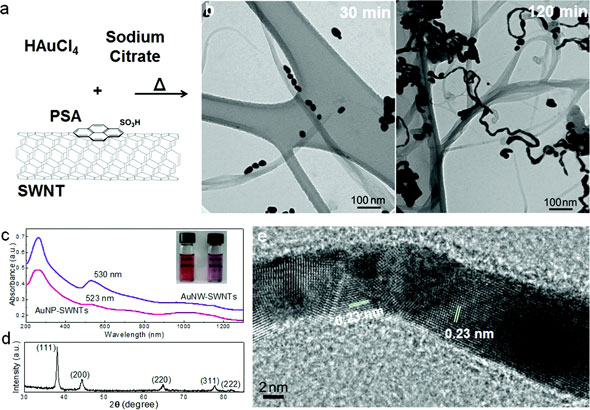
Researchers at the University of Pittsburgh have coaxed gold into nanowires as a way of creating an inexpensive material for detecting poisonous gases found in natural gas. Along with colleagues at the National Energy Technology Laboratory (NETL), Alexander Star, associate professor of chemistry in Pitt's Kenneth P. Dietrich School of Arts and Sciences and principal investigator of the research project, developed a self-assembly method that uses scaffolds (a structure used to hold up or support another material) to grow gold nanowires. Their findings, titled “Welding of Gold Nanoparticles on Graphitic Templates for Chemical Sensing,” were published online Jan. 22 in the Journal of the American Chemical Society.
“The most common methods to sense gases require bulky and expensive equipment,” says Star. “Chip-based sensors that rely on nanomaterials for detection would be less expensive and more portable as workers could wear them to monitor poisonous gases, such as hydrogen sulfide.”
Star and his research team determined gold nanomaterials would be ideal for detecting hydrogen sulfide owing to gold’s high affinity for sulfur and unique physical properties of nanomaterials. They experimented with carbon nanotubes and graphene—an atomic-scale chicken wire made of carbon atoms—and used computer modeling, X-ray diffraction, and transmission electron microscopy to study the self-assembly process. They also tested the resulting materials’ responses to hydrogen sulfide.
“To produce the gold nanowires, we suspended nanotubes in water with gold-containing chloroauric acid,” says Star. “As we stirred and heated the mixture, the gold reduced and formed nanoparticles on the outer walls of the tubes. The result was a highly conductive jumble of gold nanowires and carbon nanotubes.”
To test the nanowires’ ability to detect hydrogen sulfide, Star and his colleagues cast a film of the composite material onto a chip patterned with gold electrodes. The team could detect gas at levels as low as 5ppb (parts per billion)—a detection level comparable to that of existing sensing techniques. Additionally, they could detect the hydrogen sulfide in complex mixtures of gases simulating natural gas. Star says the group will now test the chips’ detection limits using real samples from gas wells.
Also involved in the study were Dan Sorescu, research physicist at NETL, who performed computational modeling of the gold nanowire formation; Mengning Ding, a Pitt graduate student in chemistry, who performed experimental work and synthesized and characterized gold nanowires and measured their sensor response; and Gregg Kotchey, a fellow Pitt graduate student in chemistry, who synthesized some of the graphene templates used in this study.
Funding for this work was provided by NETL in support of ongoing research in sensor systems and diagnostics.
Source: University of Pittsburgh
Additional Information:
0 comments:
Post a Comment